Mermaid Medical Group® Achieves Zero Non-Conformities in ISO 14001 Environmental Audit
Mermaid Medical Group® is proud to share that it has successfully passed its ISO 14001 audit with zero non-conformities identified.
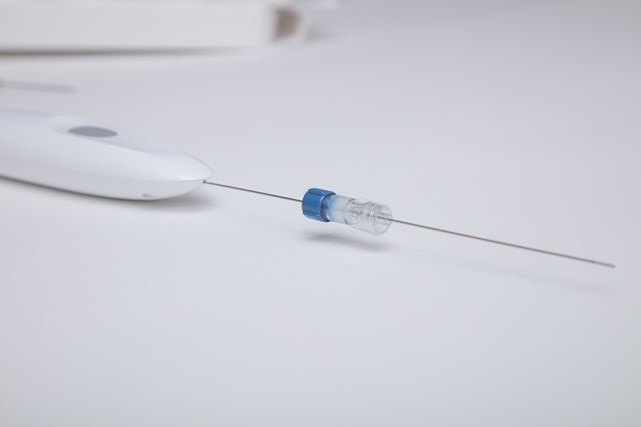
Earlier this year, we announced our exclusive distribution deal with Single Pass for their Kronos biopsy closure device, and with its recent FDA clearance, Mermaid Medical is ready to introduce the innovative cauterization device to U.S. healthcare professionals!
“I am thrilled that we are now able to bring the Kronos biopsy closure device to the U.S. market. This is a big step towards improving biopsy procedures and patient safety even further. We may also see a decrease in the need for post-procedure observation, making it a cost-effective solution as well.”
- Lars Vinther, CEO of Mermaid Medical.
The Innovative Post Biopsy Solution
The Kronos biopsy closure device is a single-use, electrocautery device that can cauterize deep tissue through a guide needle, making it a safe and effective solution to help reduce the risks of hemorrhage. Millions of people go through solid organ biopsy procedures each year, and being able to effectively seal biopsy channels can help lower the need for open surgical repair, blood transfusions, and extended hospital stays.
A Special Collaboration
We are proud of our partnership with Single Pass, and being their commercial partner and distributor on the U.S. market perfectly aligns with our commitment to help saving and improving patient lives. With Single Pass’ innovative cauterization device and our extensive distribution capabilities we are embarking on an important and exciting journey together.
To quote dedicated Single Pass CEO Bill Colone: “Together, we aim to revolutionize biopsy closure procedures and improve patient outcomes.”
About Single Pass
Single Pass is a medical device company committed to developing innovative solutions for post-biopsy bleeding, enhancing patient care, and improving clinical outcomes. Having developed a patented battery-powered electrocautery device for cauterizing deep tissue biopsy channels following solid organ procedures, Single Pass aims to ensure easy and safe biopsy procedures. Headquartered in California, USA, Single Pass is at the forefront of medical technology advancements.
Mermaid Medical Group® is proud to share that it has successfully passed its ISO 14001 audit with zero non-conformities identified.
Mermaid Medical Group® is pleased to announce an exclusive distribution agreement with MicroPort® for two cardiovascular devices: the VitaFlow Liberty™ Aortic Valve & Delivery System and Anchorman™ for LAAC procedures.
Mermaid Medical Group is excited to introduce its latest product addition: the Full Core Biopsy Device - designed with both optimal biopsy sample quality and user ergonomics in mind.